When a patient tests positive for measles, pertussis, or one of the other 120+ reportable diseases, a series of reports are initiated. Clinicians notify their local or state health department, which then reports the case to CDC through the National Notifiable Disease Surveillance System (NNDSS).
This system has been around in some form in the United States for over 150 years, going back to 1874. It's the backbone of how the United States tracks infectious disease trends at the national level, and it is one of the ways we monitor disease trends over the long term, like the dramatic reduction in measles following the introduction of a vaccine.
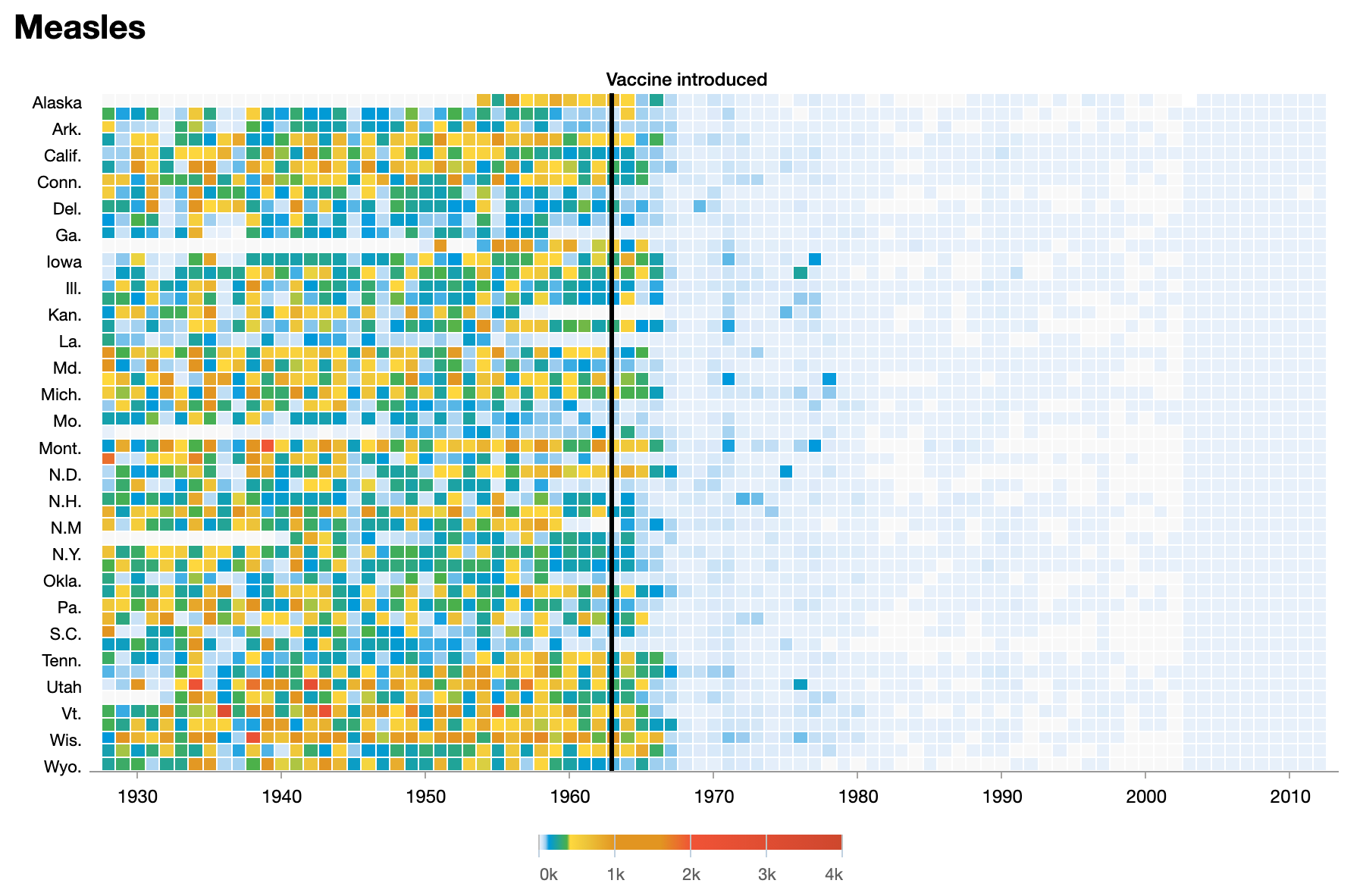
The problem is that the data is hard to access and use, stuck in a combination of PDF tables and complex online databases. For busy clinicians who just want the latest trends, there's no easy way to get a quick answer about what's going around.
At FOI Clinical, we're building data infrastructure to monitor NNDSS data and other disease surveillance sources so we can deliver timely, plain-language updates on key reportable diseases, including:
- Vaccine preventable diseases like measles and pertussis
- Invasive group A strep
- Dengue and other emerging arboviruses
- and more
Clinicians should not have to dig through surveillance reports to know when something new or important is circulating. We'll do that work and tell you what matters.
Why a paid product?
Over the years, academic researchers have built tools to make NNDSS and other disease surveillance data more accessible. These projects do good work, but they eventually end when the grant runs out. Moreover, projects are rarely funded to communicate the data to a wide audience, so the data is rarely put to best use.
We're building FOI Clinical as a subscription product because we want it to last. Subscriber support means we're not dependent on grant funding. It also means we answer to users rather than to funders with evolving priorities.
More updates coming soon as we build toward our February 2026 launch. Thank you for your early interest. If you want to support our work ahead of launch, individual and group pre-launch subscriptions are now available.

